Don’t let non-RCRA pharmaceutical waste become a compliance nightmare. Learn the crucial differences between hazardous waste pharmaceuticals and regulated medical waste to ensure proper disposal and reduce costs for your health care facilities.
You’ve got a cabinet full of discarded pharmaceutical products, and you need to ensure every last vial and expired pill ends up in the correct container. It’s a perplexing problem staff face daily in med spas, outpatient surgical centers, and pharmacies.
Misclassification of pharmaceutical waste isn’t just a minor mistake. Improper disposal can significantly drive up your operational costs and expose your healthcare facilities to substantial compliance risks.
You work hard to maintain an efficient, compliant practice. Let’s simplify your waste management by first breaking down the key differences between the two major waste streams: regulated medical waste (RMW) and non-RCRA pharmaceutical waste.
What is Regulated Medical Waste (RMW)?
Regulated medical waste (also known as medical waste or biohazardous waste) is anything that is, or has the potential to be, infectious. The Centers for Disease Control and Prevention (CDC) and the Environmental Protection Agency (EPA) definitions focus on materials that may transmit pathogenic organisms.
If it’s been contaminated with blood, other potentially infectious materials (OPIM), or sharp items, it generally belongs in the RMW stream.
This waste is not considered hazardous in the RCRA (Resource Conservation and Recovery Act) sense, but it still poses health risks through infection if not handled correctly by healthcare professionals.
Common RMW Examples for Your Facility:
- Sharps: Used needles, syringes, disposable scalpels, and broken glass that have been in contact with patient materials.
- Contaminated Items: Gauze, bandages, sponges, and disposable PPE soaked or caked with blood or OPIM.
- Vials: Vials that previously held vaccines, injectables, or pharmaceuticals, once they are empty or have trace residue and are contaminated with patient fluid or blood.
Proper RMW disposal is all about safety and preventing the spread of infection. It is essential for every medical facility handling waste to comply with regulations.
Blue Bin Best Practices: Non-RCRA Pharmaceutical Waste
The Resource Conservation and Recovery Act (RCRA), also known as the Recovery Act, is the federal law governing hazardous waste and hazardous chemicals in the United States.
When a pharmaceutical product is not listed or identified as a hazardous waste under RCRA, it is classified as non-RCRA pharmaceutical waste. These are also frequently called non-hazardous pharmaceutical waste or non-hazardous pharma waste.
This category includes the majority of expired, unused, or dispensed pharmaceuticals that are not infectious and are not hazardous pharmaceutical wastes.
This non-RCRA waste material still requires proper pharmaceutical waste disposal to prevent environmental contamination and keep it out of the water supply, but is much less complex to manage.
Common Non-RCRA Pharma Waste Examples:
- Expired or Unused Medications: Pills, capsules, and non-RCRA liquids that are past their use date or have been partially dispensed and need disposal. These can also be referred to as non-hazardous waste.
- Trace Chemotherapy Waste: Items that have trace amounts (not bulk quantities) of chemotherapy drugs, like empty IV bags and tubing or lightly contaminated PPE.
- Non-Hazardous Vials: Injectable vials, ampoules, and pre-filled syringes that are unused or have expired drugs and are not contaminated with blood or patient material.
- Non-RCRA Drugs: Certain drugs categorized as non-hazardous, often due to their low toxicity profile or low volume of hazardous chemicals.

Image Source: Shutterstock
What About the Truly Toxic RCRA Hazardous Waste?
While most of your facility’s discarded pharmaceuticals fall into the non-hazardous pharmaceutical waste stream, a significant, separate category exists: RCRA pharmaceutical waste.
Hazardous pharmaceutical wastes must be managed under strict EPA regulations for waste disposal. These are pharmaceuticals considered hazardous under the RCRA because they meet one of two criteria:
- Characteristic Hazardous Waste (D-list): The drug is ignitable (e.g. has low flash points), corrosive, reactive, or toxic. For example, a drug that is highly acidic and can damage steel.
- Listed Hazardous Waste (P- and U-lists): These are specifically named commercial chemical products. P-listed wastes are acute hazardous wastes (e.g. nicotine), even in small quantities, and U-listed wastes are non-acute hazardous wastes.
Proper management of these hazardous pharmaceuticals is paramount to prevent environmental contamination. This is the most crucial part of pharmaceutical waste management.
A Simple Decision Tree for Waste Streams
When you’re standing over a discarded item, use this simple flowchart to ensure non-hazardous pharmaceutical waste, hazardous waste pharmaceuticals, and other controlled substances meet regulatory compliance:
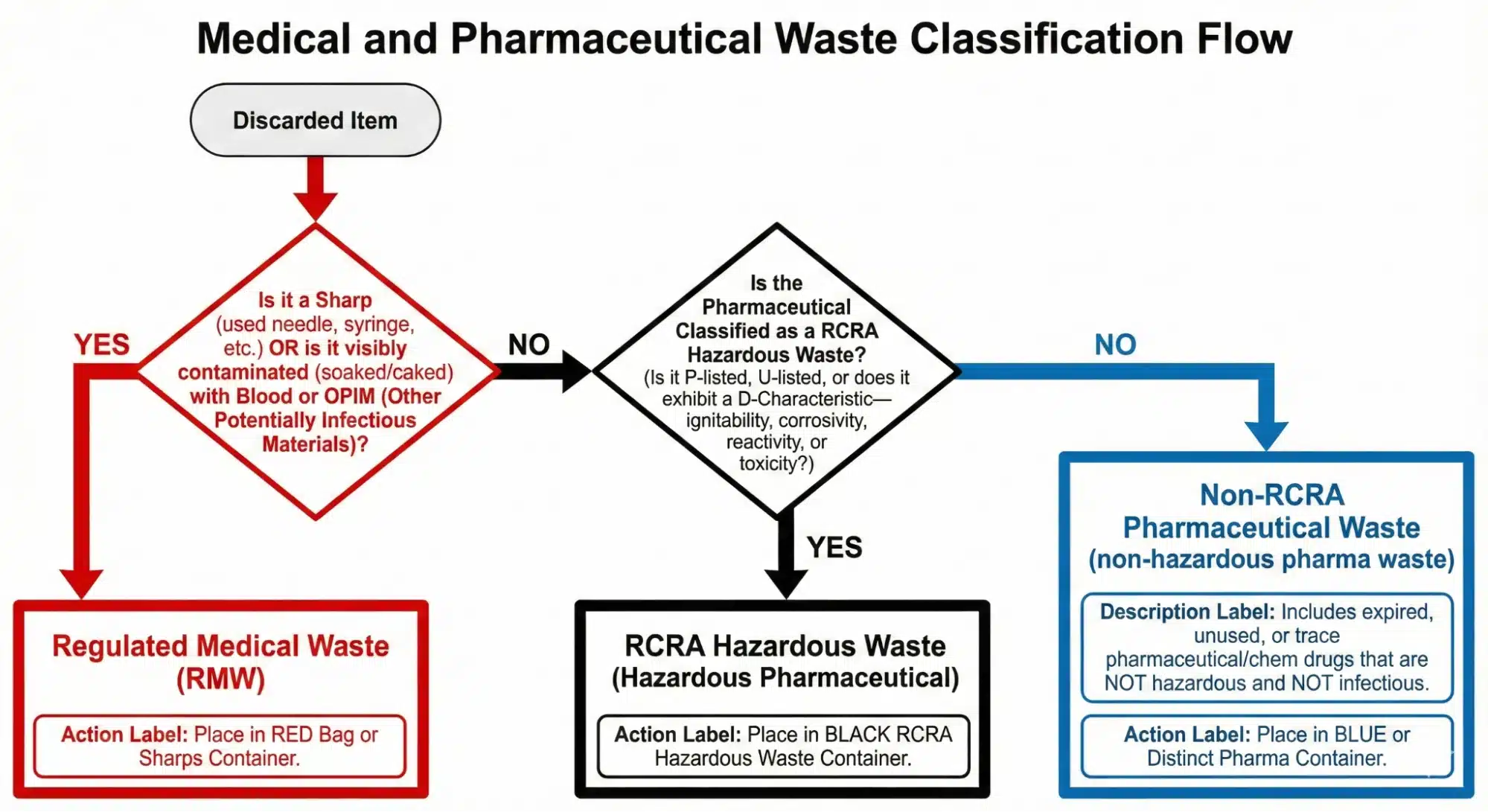
Image Source: AI-Generated
This streamlined approach helps your facility adhere to all federal regulations for disposal methods.
The Price of Precision: Why Classification Matters
Why go through the trouble? Misclassification drives unnecessary cost and risk.
- Cost Crunch: Regulated medical waste is often your most expensive stream to dispose of. Putting non-hazardous pharmaceutical waste into a red bag means paying a premium for a service you don’t need, unnecessarily inflating your costs. Furthermore, your hazardous waste requires specialized handling far more costly than disposing of non-hazardous materials.
- Compliance Calm: Sorting correctly protects your healthcare facilities. Failure to separate different waste streams can lead to fines and operational issues, especially during an inspection. Improper disposal and mixing of waste pose health risks to the public and staff.
Learn more: Why Effective Medical Waste Solutions Are Essential for Healthcare Safety
FAQs about Non-RCRA Pharmaceutical Waste
This non-hazardous pharmaceutical waste is collected in distinct containers, typically colored blue or designated black/white bins, separating it from red-bagged RMW and black RCRA hazardous waste.
Hazardous waste is defined by the RCRA (P, U, D lists) due to properties like toxicity or corrosivity. Non-hazardous pharmaceutical waste does not meet these federal criteria, though it still requires specific disposal methods.
Separating unused medications from medical waste protects public health, prevents harmful substances from interacting with the environment, and ensures your healthcare facilities remain compliant.
Yes. Items with only trace amounts of chemotherapy drugs, such as empty IV bags or tubing, are generally managed as non-RCRA pharmaceutical waste. Bulk quantities, however, are often hazardous pharmaceutical wastes.
Key Takeaways
- Non-hazardous pharmaceutical waste (often blue-binned) includes most expired or unused non-infectious drugs.
- Regulated Medical Waste (RMW) goes into red containers and covers items contaminated with blood or other infectious materials.
- RCRA pharmaceutical waste is legally defined hazardous waste requiring specific black container management due to toxicity or other dangerous characteristics.
- Misplacing non-hazardous pharmaceutical waste into RMW containers unnecessarily increases your facility’s disposal costs.
- Accurate separation into correct waste streams helps your healthcare facility maintain regulatory compliance and mitigates potential health risks.
Complex Waste Streams, Simplified Solutions
Biogenic Solutions knows that no two facilities are alike. Whether you run a med spa or an outpatient center, managing complex streams like RCRA, non-RCRA, and RMW is a challenge, but it doesn’t have to be yours.
We provide more than just disposal. We act as your dedicated compliance partner. Enjoy simplified logistics, reliable scheduled pickups, and transparent volume-based pricing tailored specifically to your facility’s needs.
Ready to streamline your disposal processes and save on costs? Learn more about our regulated medical waste service and how we can become your compliance partner today.